|

Gaithersburg-based biotech MedImmune has appointed Yong-Jun Liu, chief scientific officer of the Baylor Research Institute, as its new head of research.
The appointment puts Liu in a pivotal role, not just for MedImmune but also for its parent company, AstraZeneca, which is depending on its U.S.-based biologics arm to supply a pipeline of early-stage drug candidates.
back to top 
 The University of Maryland on Wednesday announced the appointment of Thomas R. Fuerst, Ph.D., as the new director of the Institute for Bioscience and Biotechnology Research. The University of Maryland on Wednesday announced the appointment of Thomas R. Fuerst, Ph.D., as the new director of the Institute for Bioscience and Biotechnology Research.
IBBR is a joint research enterprise created to enhance collaboration among the University of Maryland, College Park (UMD), the University of Maryland, Baltimore (UMB), and the National Institute of Standards and Technology (NIST) in the fields of medicine, biosciences, technology, quantitative sciences and engineering.
back to top 

Physician’s Choice Laboratory Services (PCLS) announced today that it has recently partnered with Genesys Biolabs, a division of 20/20 GeneSystems, Inc. (Rockville, MD) to offer PAULA’s Test (Protein Assay Using Lung Cancer Analytes), a simple blood test that aids physicians in the early detection of lung cancer.
“As an enthusiastic proponent of personalized medicine, PCLS is pleased to partner with 20/20 GeneSystems to promote their newest assay for early stage detection of lung cancer, PAULA’s Test. This test will be of great value to the physicians and patients affected in communities PCLS serves. Lung cancer is a curable disease when caught early and risk-directed screening will save lives as well as reduce the total cost of treatment. The test is a beneficial leap forward for high-risk individuals, providers, and the healthcare system,” said Joe Wiegel, President of PCLS.
back to top 

A new interdisciplinary center at the University of Maryland is at the forefront of a digital revolution in health care.
back to top 

Johns Hopkins undergraduate students have invented a system to shock a dangerously irregular heart back into normal rhythm more safely and effectively.
The two-component system is designed both to expand a doctor's options in routing electric current through the heart and to improve the application of pressure to the patient's body to help treatment succeed.
back to top 

Two more venture capital firms have been selected to receive InvestMD funds that they will invest in local startup companies.
EnerTech Capital Partners will receive $10 million and Foundation Medical Partners will receive $7 million. As part of the state’s InvestMaryland program, the firms will use it to back local startup companies. After a startup exits, the state gets 100 percent of its principal investment and 80 percent of the proceeds from the exit. The venture firms can keep the remaining 20 percent of the proceeds.
back to top 

New Enterprise Associates has had a hand in a number of Boston’s biotech startups over the years. But it wasn’t until now that the big VC firm officially put a physical footprint in the biotech cluster in Cambridge, MA.
NEA today is announcing that it has opened an office in Kendall Square. It’s on the third floor at 700 Tech Square in Cambridge, and will serve as a local home base for the VC firm and its healthcare partners, many of which serve on boards in the area. NEA already has offices in New York, California, Washington, D.C, Chicago, China, and India.
back to top 

QIAGEN (NASDAQ: QGEN; Frankfurt, Prime Standard: QIA) today announced an agreement with Eli Lilly and Company (NYSE: LLY) to develop and commercialize a molecular companion diagnostic paired with a novel Lilly oncology compound. This is the third co-development project by QIAGEN and Lilly to create companion diagnostics, which are tests that analyze genomic information in patient samples to enable personalized decisions on treatments. The latest collaboration, involving an undisclosed Lilly compound and an undisclosed molecular diagnostic target, builds on a master collaboration agreement for development of tailored therapies in cancer and other therapeutic areas signed earlier this year.
QIAGEN and Lilly are long-standing partners in personalized healthcare. QIAGEN’s therascreen® KRAS RGQ PCR Kit has been widely adopted by laboratories since its July 2012 approval by the Food and Drug Administration (FDA) as a companion diagnostic. The therascreen KRAS Test detects gene mutations in metastatic colorectal cancer patients, indicating which ones will benefit from Erbitux. In September 2011, QIAGEN and Lilly partnered to develop a companion diagnostic that evaluates the Janus kinase 2 (JAK2) gene, which plays a role in some blood cancers. The test is paired with a Lilly compound to guide use of the proposed drug, currently in clinical trials.
back to top 

GlaxoSmithKline and Theravance's new inhaled lung drug Relvar has been approved in Europe to treat both asthma and chronic obstructive pulmonary disease (COPD), confirming an endorsement from regulators in September.
The medicine, which is inhaled through a palm-sized device called Ellipta, consists of a corticosteroid to reduce inflammation and a novel long-acting beta-agonist (LABA), which is designed to open the airways.
back to top 

A Cambridge company developing new drugs focused around DNA damage and genetically defined cancers has attracted investment from another pharma giant, Pfizer, in its latest funding round, a £20 million Series B.
Pfizer Venture Investments was the only new investor in Mission Therapeutics’ Series B round, which was led by existing investor Sofinnova Partners and also included Imperial Innovations, SR One and Roche Venture Fund, which means it now has three major pharmaceutical companies backing it – SR One is GlaxoSmithKline’s corporate healthcare VC fund.
back to top 

Johns Hopkins is more than halfway to its $4.5 billion fundraising goal, the university announced Wednesday, with the money helping to support initiatives that include urban revitalization and global health.
More than 162,000 donors have helped Hopkins meet the halfway mark earlier than officials had previously expected, in spring 2014. The $4.5 billion fundraising goal is among the biggest such efforts in the country and the largest for the Johns Hopkins University and Johns Hopkins Hospital.
back to top 

The Professor Venture Fair began at Bioscience Day 2007 and has become an annual event that gives faculty inventors the opportunity to pitch their new technologies to a team of venture capitalists and entrepreneurs from the region. Presenters are judged based upon clarity of pitch and commercial viability.
Bioscience Day 2013 Venture Fair 11:00 am - 12:00 n
Moderator: Gayatri Varma, Director, OTC
Presenters and INventors: Anthony Melchiorri and John Fisher; Yanjin Zhang; Hadar Ben-Yoav, Reza Ghodssi, Gregory Payne and Deanna L. Kelly ;Kenyon Crowley, Ritu Agarwal , Guodong "Gordon" Gao, Nanette I Steinle and Arnab Ray; Donald DeVoe
Judges:Todd Chappell, Wyatt Somogyi, Matt Cohen, Stephen P. Auvil
Location: Grand Ballroom Lounge, Stamp Student Union
back to top 

Theme: "The First Mile of a Marathon". The theme explores the next steps towards commercialization of University developed technologies. Most inventors painstakingly develop their technologies, and yet, underestimate the herculean task of moving an idea from the lab to launch. Even before funding, there is a lot of heavy lifting, analysis, customer discovery, team building, etc. that needs to be done. The panel speakers will discuss these next steps that every inventor or potential entrepreneur has to undertake.
Moderator: Elana Fine, Managing Director, Dingman Center for Entrepreneurship
Panelists: Todd Chappell, Wyatt Somogyi, Matt Cohen, Stephen P. Auvil
Location: Grand Ballroom Lounge, Student Stamp Union, UMCP
back to top 

The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notice:
NOT-HL-13-200: Clarification of Number of Applications to RFA-HL-14-028 "Blood and Vascular Systems Response to Sepsis (R01)"
Requests for Applications (RFAs):
RFA-HL-14-020: Evaluation and Administration Coordinating Center for the Low-Cost, Pragmatic, Patient-Centered Randomized Controlled Intervention Trials (R01)
This NIH Funding Opportunity Announcement (FOA) invites applications to support an Evaluation and Administration Coordinating Center. This unit will facilitate the coordination among and between the awardees of RFA-HL-14-019 "Low-Cost, Pragmatic, Patient-Centered Randomized Controlled Intervention Trials (UH2/UH3)" and the NIH. This unit will also be responsible for conducting an evaluation of the RFA-HL-14-019 program.
RFA-HL-14-019: Low-Cost, Pragmatic, Patient-Centered Randomized Controlled Intervention Trials (UH2/UH3)
This NIH Funding Opportunity Announcement (FOA) invites applications to plan and conduct low-cost, pragmatic randomized controlled trials (RCTs).
back to top 

Using scores obtained from cognitive tests, Johns Hopkins researchers think they have developed a model that could help determine whether memory loss in older adults is benign or a stop on the way to Alzheimer's disease.
The risk of developing dementia increases markedly when a person is diagnosed with mild cognitive impairment, a noticeable and measurable decline in intellectual abilities that does not seriously interfere with daily life. But physicians have no reliable way to predict which people with mild cognitive impairment are likely to be in the 5 to 10 percent a year who progress to dementia.
back to top 

Mary Washington Healthcare (MWHC) and Zebrareach today announced a collaboration that provides MWHC's more than 4,000 Associates free membership to Zebrareach, a smartphone application that gives employees loyalty discounts at local stores, as well as other special offers negotiated for MWHC. The Zebrareach membership program for MWHC will launch in November 2013.
Zebrareach (http://zebrareach.com) is a mobile customer engagement application for small business that builds customer retention through a free consumer smartphone application. Businesses build and manage volume and tier-based loyalty programs, message exclusive news, product announcements, special events, secret menu items, limited time offers and more to their loyal customers. Customers are able to place orders for products and services through the web and mobile Zebrareach application. Zebrareach works for all customers, with or without mobile phones.
back to top 
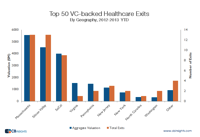
An earlier research brief we’d issued highlighted that Silicon Valley dominates the list of top 50 VC-backed tech exits. The post generated a fair amount of chatter including some comments calling us arrogant Silicon Valley’ites (we’re based in NYC).
Among the more constructive comments were several from healthcare VCs who wondered if the data would be similar for the healthcare sector. The prevailing hypothesis was that Silicon Valley’s dominance wouldn’t translate to healthcare (or perhaps not as much). Note: our healthcare classification includes companies ranging from medical devices to biotech to pharma.
back to top 

Billlionaire investor Mark Cuban, who owns the Dallas Mavericks and Landmark Theaters and is a regular on Shark Tank, has expanded his interests to personalized health. He is part of a group of investors in mobile health startup, Validic,which raised $760,000 in a seed round. The Durham, North Carolina company has developed a platform to integrate and aggregate data from more than 80 health-oriented apps.
Cuban said this about the investment:
“Personalized health is the future of healthcare…and with the explosive growth of new mobile apps and devices coming on the market, Validic solves a fundamental problem of integrating all those new innovations into the healthcare system. I’m very excited for the future of Validic and the mobile health space.”
back to top 

In the past few years, pharmaceutical distributor Cardinal Health (NYSE:CAH) has acquired a major distributor and half a dozen pharmaceutical companies in China. It also acquired AssuraMed to enter the home-health supply market, and invested in startups including HealthSpot, which makes a telemedicine kiosk, and Intralign, which helps providers optimize the cost and quality of surgical care.
Those moves were all part of a clearly defined strategy to meet the changing healthcare marketplace. John Rademacher, president of ambulatory care at Cardinal Health, told BioOhio members at the trade group’s annual event on Tuesday that the company’s investment and acquisition strategy is driven by these 12 healthcare industry trends:
back to top 

Big data may be about to overwhelm the healthcare system. A little healthcare business intelligence tip: Data by itself won’t drive value and outcomes. Smart healthcare analytics will. In Deloitte’s DBrief, “Big Data Revolution: Unlocking Healthcare Analytics,” healthcare industry experts talked about the opportunities and barriers for industries across the care continuum to harness data, contextualize it and use it to move from hindsight to insight (and eventually, with the help of predictive analytics, foresight).
“The future is already here,” Brett Davis, a principal at Deloitte, said. “It just hasn’t been evenly distributed yet.” Here are the six key trends in healthcare shaping how data will be used.
back to top 
Much has been written about the pharmaceutical industry’s R&D-productivity challenge during the past decade: the decline in new-drug approvals has raised discovery and development costs just as companies struggle to find new drugs to replace blockbusters that have lost (or will soon lose) their exclusivity. Yet by one important measure, the output of the pharmaceutical R&D process has accelerated significantly: the US Food and Drug Administration (FDA) approved 39 new drugs in 2012—the highest level in a decade.
back to top 

We’ve heard a lot this year about the IPO boom for biotech companies. Even after a few high-profile blowups (Ariad, Sarepta), the public biotech stock indexes are still outperforming the Nasdaq Composite Index and S&P 500. Some biotechs have been acquired for megabucks (Onyx, ViroPharma). We’ve heard about another biotech bubble in the making.
back to top 

Apply Now to be a Presenting Company at Bio€quity Europe 2014
Now celebrating its 15th meeting, Bio€quity Europe is the premier industry event for financial dealmakers looking for investor-validated life science companies positioning themselves to attract capital and for pharma licensing professionals to assess top biotech prospects. Bio€quity Europe has showcased more than 600 leading European companies to thousands of investment and pharma business development professionals. Delegates from over 20 nations attended Bio€quity Europe last year.
Present Your Story to the Financial Community
Each Presenting Company provides a thorough 25-minute overview to fund managers, venture capitalists and pharma business development and licensing professionals.
Special "Next Wave" sessions feature young innovator companies and consist of eight-minute presentations on the company, technology and programs. In addition, the turf-neutral setting provides unique access to a cross-section of sellside analysts, investment bankers, and business development professionals from top-tier pharmaceutical and biotech companies in a single location.
Special events and private meeting space allow Presenting Companies, "Next Wave" presenters and Sponsors to network and conduct one-on-one meetings with the delegates throughout the two-day event.
Contact: conferences@biocentury.com
back to top 

Last week, the nation’s leading heart organizations released a sweeping new set of guidelines for lowering cholesterol, along with an online calculator meant to help doctors assess risks and treatment options. But, in a major embarrassment to the health groups, the calculator appears to greatly overestimate risk, so much so that it could mistakenly suggest that millions more people are candidates for statin drugs.
The apparent problem prompted one leading cardiologist, a past president of the American College of Cardiology, to call on Sunday for a halt to the implementation of the new guidelines.
back to top 

A patient with abdominal pain dies from a ruptured appendix after a doctor fails to do a complete physical exam. A biopsy comes back positive for prostate cancer, but no one follows up when the lab result gets misplaced. A child’s fever and rash are diagnosed as a viral illness, but they turn out to be a much more serious case of bacterial meningitis.
Such devastating errors lead to permanent damage or death for as many as 160,000 patients each year, according to researchers at Johns Hopkins University. Not only are diagnostic problems more common than other medical mistakes—and more likely to harm patients—but they’re also the leading cause of malpractice claims, accounting for 35% of nearly $39 billion in payouts in the U.S. from 1986 to 2010, measured in 2011 dollars, according to Johns Hopkins.
back to top 

IBM has made its Watson cognitive computing technology available as a cloud-based app development platform, and healthcare vendors are already getting in on the act.
Company officials they hope to encourage new uses of the fast-evolving technology and spur a slew of innovative apps. In this new marketplace, they say, developers of all sizes and industries can access resources – developer toolkits, educational materials and access to Watson's application programming interface – for developing Watson-powered technology of their own.
back to top 

Mark Cuban’s comments about personalized health may have turned a few heads but as one source soberly reminded us, he is not the first or the only billionaire investor in healthcare. Not by a long shot. Several people who have nine zeroes in their net worth have invested in the space. They’re motivated by emerging mobile health technology to help reduce healthcare costs and see it as playing a critical role in the future of healthcare technology. There are also philanthropic considerations to increase access to healthcare in underserved populations.
MhealthInsight reckons there are about 19 billionaire investors in mobile health, including Cuban. Here are some highlights from that list.
back to top 
For budding startups, accumulating funding is necessary — but difficult. Crowdfunding can be a viable alternative for entrepreneurs.
That was one key takeaway for the few hundred part-time and aspiring entrepreneurs gathered at the Entrepreneurs Inspiring Entrepreneurs Expo at the BWI Marriott Monday who caught the “Sourcing the Crowd” panel discussion.
back to top 

Paul Silber had some unexpected advice from a venture capitalist for the entrepreneurs who crowded a conference room at the BWI Airport Marriott Monday hoping to find out how to land some VC cash.
Silber’s suggestion: tap all other sources first, like friends and family and angel investors, before looking to a venture capital firm for funding.
back to top 

We’ve all done it. You throw your clothes in a bag and head to the airport. Sixteen hours later, you’re in a country where the customs, dress, language and food are very different from home. As you leave the airport, you stop at an ATM, and within seconds have enough local currency for a taxi and a few meals. All you needed was an ATM card and some money in the bank.
In fact, your trip is going really well until you slip on some ice and fall down a flight of stairs. As you tumble to the bottom and see your femur bone break through the skin, you wonder whether you will be awake to tell the hospital about your allergy to local anesthetics and your heart disease, which has left you with an abnormal heart rhythm.
back to top 
|