|

Immunotherapy is at the forefront of cancer care; however, most cancers evolve the ability to dodge the body’s defenses.
Maryland startup BeneVir is developing immunotherapy viruses that rid the body of two types of tumor cells – those that cause cancer and the ones that make it recurrent.
back to top 

British drugmaker AstraZeneca plans to spend $200 million over the next three years, expanding its manufacturing facility in Frederick, Md., and hiring an additional 300 workers at the site, executives said.
The decision further cements Gaithersburg-based MedImmune, an AstraZeneca company, as the crown jewel of Maryland’s life sciences industry.
back to top 

An experimental vaccine to prevent Ebola virus disease was well-tolerated and produced immune system responses in all 20 healthy adults who received it in a Phase 1 clinical trial conducted by researchers from the National Institutes of Health. The candidate vaccine, which was co-developed by the NIH's National Institute of Allergy and Infectious Diseases (NIAID) and GlaxoSmithKline (GSK), was tested at the NIH Clinical Center in Bethesda, Maryland. The interim results are reported online in advance of print in the New England Journal of Medicine.
back to top 

The stampede is back on among venture capital firms to raise new money and close more funds, after years of standing pat following the recession and years of sluggish recovery.
back to top 

That next blockbuster drug? It all begins with a hypothesis: GlaxoSmithKline just announced the winners of its second Discovery Fast Track Challenge – a competition that teams up American and European academia with GSK researchers to speed up their search for new therapeutics.
back to top 

The Washington Business Journal interviewed Leslie Ford Weber, JHU's director of the Montgomery County Campus and of government and community affairs for Montgomery County. The feature ran as an Executive Profile on Nov. 14, 2014. It was written by Vandana Sinha, an assistant managing editor at the Washington Business Journal. The photo was taken by Joanne S. Lawton.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
Notices:
- NIH Implementation of the US Government Policy on Institutional Oversight of Life Sciences Dual Use Research of Concern
- Publication of Notice of Proposed Rulemaking for Clinical Trials Registration and Results Submission under FDAAA
- NIH Request for Public Comments on the Draft NIH Policy on Dissemination of NIH-Funded Clinical Trial Information
- Request for Information (RFI): Inviting Comments and Suggestions on the Reagent-Related Barriers to Reproducible Research
- Reminder: Annual Reports to the Office of Laboratory Animal Welfare due January 31, 2015
- Notice of Intent to Publish a Funding Opportunity Announcement for Sudden Death in the Young: Population-Based Studies (U01)
Program Announcements
- Systems Science and Health in the Behavioral and Social Sciences (R21)
- (PAR-15-047)
- Office of Behavioral and Social Science Research
- National Cancer Institute
- National Institute on Aging
- National Institute on Alcohol Abuse and Alcoholism
- National Institute of Biomedical Imaging and Bioengineering
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
- National Institute of Dental and Craniofacial Research
- National Institute of Environmental Health Sciences
- National Institute of Mental Health
- National Institute of Nursing Research
- Office of Disease Prevention
- Application Receipt/Submission Date(s): Multiple dates, see announcement.
- Systems Science and Health in the Behavioral and Social Sciences (R01)
- (PAR-15-048)
- Office of Behavioral and Social Science Research
- National Cancer Institute
- National Institute on Aging
- National Institute on Alcohol Abuse and Alcoholism
- National Institute of Biomedical Imaging and Bioengineering
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
- National Institute of Dental and Craniofacial Research
- National Institute of Environmental Health Sciences
- National Institute of Mental Health
- National Institute of Nursing Research
- Office of Disease Prevention
- Application Receipt/Submission Date(s): Multiple dates, see announcement.
back to top 

In 2015, hospitals will – and should – make more advanced use of "third platform" technologies based on mobile tools, social channels, data analytics and the cloud, according to a recent report from IDC Health Insights.
With healthcare costs unsustainable, but these new technologies now ubiquitous, IDC officials say hospital CIOs will increasingly be turning to new tools – especially as consumers expect healthcare to be as responsive to their wants and needs as other industries.
back to top 

Angel investors are often rich individuals who provide startups with capital for their start-up costs. The term comes from Broadway, where it was originally used to describe the wealthy individuals who provided money for theatrical productions.
back to top 

Myron M. Levine, MD, DTPH, director of the University of Maryland School of Medicine Center for Vaccine Development (CVD), and Dean E. Albert Reece, MD, PhD, MBA, announced today the start of a clinical trial in Baltimore to evaluate different dosage levels of a promising experimental Ebola vaccine developed by the Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and GlaxoSmithKline (GSK
back to top 

The Federal Laboratory Consortium for Technology Transfer (FLC), a nationwide network of federal laboratories that cultivates best-practice strategies for advancing technology transfer (T2) from the laboratory to the marketplace, today announced the launch of FLCBusiness, an interactive business resource tool.
back to top 

Lacrosse sticks, construction models and surgical tools — these are all things Baltimore companies are making with the help of 3-D printing.
Three-dimensional printing was invented decades ago but has really taken off in the last few years. Printers are more affordable (you can get one for your desktop for the price of a MacBook Pro). And printing material has advanced significantly, to include more durable plastics, metal and more.
back to top 

Every once and a while we get a clear example of the gulf between those battling over important public policy issues and can understand why the public and policy makers are confused by resulting charges and counter charges. Last week was a good illustration.
back to top 
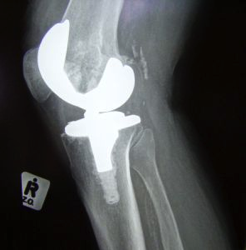
David Chalker, 50, has excruciating pain in his hip. He’s an Army veteran and because of the pain, he had to leave his job as a machinist, which left him in a great deal of debt and unable to pay for health insurance. He, his wife, and his three daughters needed to move in with his in-laws as a result.
back to top 

The color of your urine could be telling you something about your health condition. Yes, your standard yellow is where you want to be, but the different shades of the rainbow make an appearance on occasion.
back to top 

Any small business or venture capital company interested in Small Business Innovation Research (SBIR) or Small Business Technology Transfer (STTR) funding opportunities should pay close attention to the Small Business Administration's (SBA) recent request for public comments, by January 6, 2015, on data rights and Phase III funding, and a recent Government Accountability Office (GAO) report identifying the National Institutes of Health (NIH) and Department of Energy's Advanced Research Projects Agency-Energy (ARPA-E) as the two agencies presently accepting applications from majority-owned portfolio companies.
back to top 

Nestled in a quiet industrial park in Redmond, Washington, not too far from the Microsoft headquarters, is a small biotech start-up with both an interesting technology they are bringing to market, as well as a capital partner that suggests some ways in which global biotech research, venture capital and commercialization are going to change.
back to top 

Small businesses are a major driver of high-technology innovation and economic growth in the United States, generating significant employment, new markets, and high-growth industries.1 In this era of globalization, optimizing the ability of small businesses to develop and commercialize new products is essential for U.S. competitiveness and national security. Developing better incentives to spur innovative ideas, technologies, and products—and ultimately to bring them to market—is thus a central policy challenge.
back to top 
|